Brain Wiring by Nature and Nurture
Our brain is the most complicated organ in our body, which comprises billions of neurons and trillions of neuronal connections with remarkable spatiotemporal specificity. Furthermore, it also comprises similar number of glia cells and complex network of blood vessels that support neuronal networks. This highly complicated yet organized structure gives rise to all our thoughts, emotions and actions. How is such a complicated structure established during development has been a central question in neuroscience. Disruptions of brain development lead to numerous brain disorders, such as ASD, ADHD, and schizophrenia. Regulation of brain wiring occurs at different scales from genome to behavior. We aim to reveal a unifying logic from nanoscale to macroscale that underlies the brain wiring regulated by nature and nurture
How does postnatal experience regulate brain wiring?
We have been committed to the study of the molecular and cellular mechanisms by which genetic programs and postnatal experience regulate brain development and plasticity. We discovered a new and evolutionarily conserved family of cell adhesion molecules that are key regulators of the spatiotemporally specific assembly of visual and motor circuits in Drosophila. We also elucidated the spatiotemporal rules by which visual experience in early life regulates the cell-type and functional development/plasticity of neurons in the mouse primary visual cortex, and revealed that experience regulates these processes by establishing spatially graded expression of genes encoding cell adhesion molecules, which further determines cell type identity and function. Recent advances in spatial transcriptomics has revealed spatially graded gene expression and cell type continuum in the whole brain. We are setting up to study whether experience can regulate this spatial molecular and cellular organization across brain areas. Furthermore, we are trying to relate this molecular and cellular architecture to patterns of neuronal connectivity and physiology across brain regions, as well as animal behavior. Overall, these efforts will establish a research system that can offer mechanistic insights across different scales with manipulation of single variables (i.e. genetics vs envorinment). This will enable us to dig deep into clinical related findings discussed below.
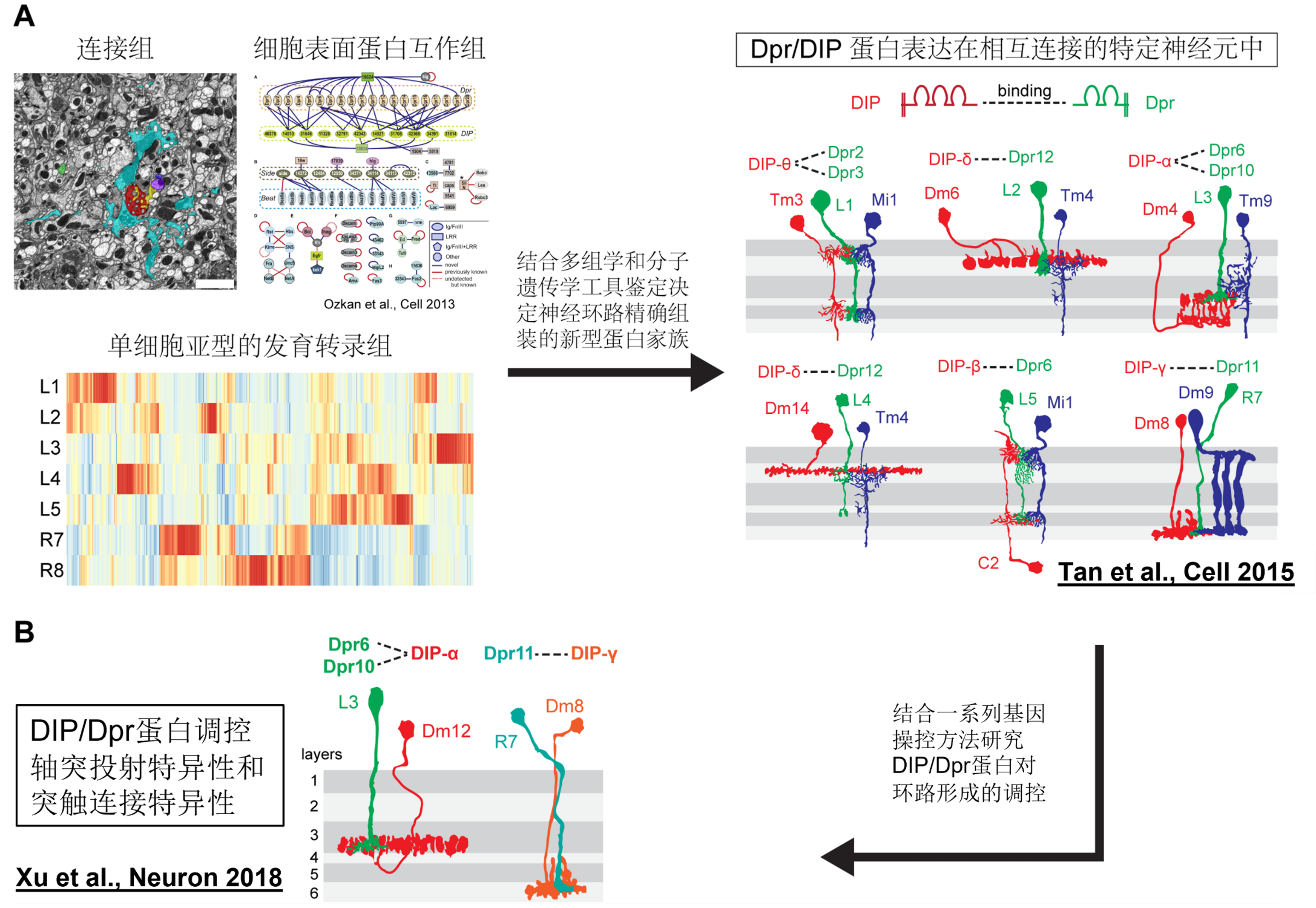
An evolutionarily conserved family of key cell recognition proteins that regulate the circuit-specific assembly of visual pathways has been identified.
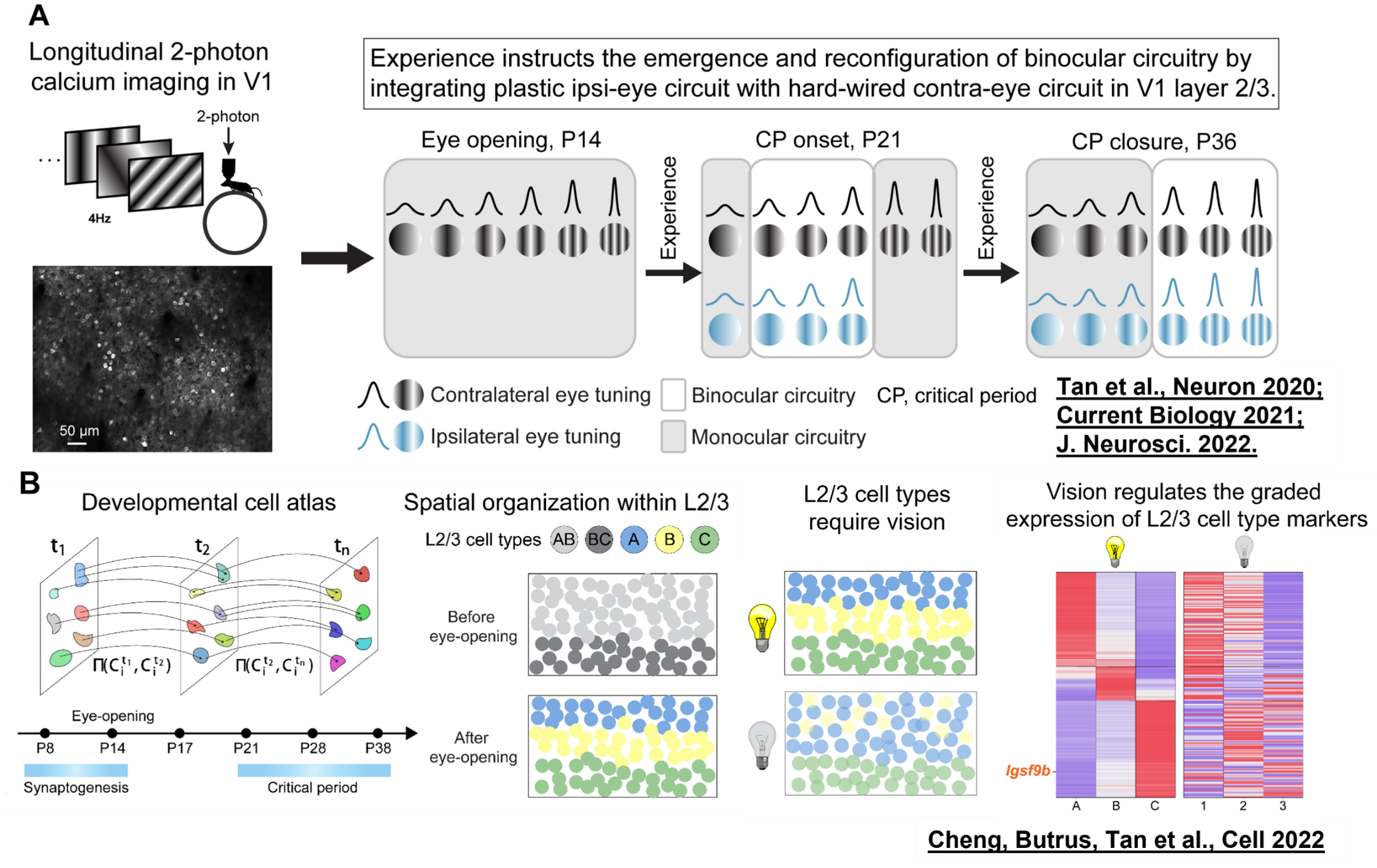
Postnatal development and plasticity of binocular circuits in the mouse visual cortex have been elucidated, revealing that experience establishes cellular subtypes and functions by regulating the spatial gradient expression of genes along the dorsoventral axis.
Genetic and environmental regulators of human brain wiring

Development of major brain functions, including sensory, motor, cognitive, social functions and personality, occurs postnatally and is regulated by both nature and nurture.
Abnormal brain development leads to neural developmental diseases such as ASD, ADHD, etc. It has been accepted that genetic and environmental factors both contribute to these diseases. However, we do not know the exact relationships between individual factors and disease phenotypes. Recent establishment of several comprehensive databases from clinical cohort studies in the US and Europe, and advances in multidimentional data analysis offer us an unprecedented opportunity to reveal quantitative insights into this question, which will further provide research objects for mechanistic studies using above mentioned cross-scale research system.
Regeneration of the visual pathway
Glaucoma and optic nerve injury are major causes of irreversible vision loss. These diseases co-feature neuronal cell death and axon loss, resemblying brain degenerative diseases such as Alzheimer’s Disease and Parkingson’s Disease. Furthermore, the eye is part of the central nervous system (CNS), although located far away from the brain. Thus, regeneration of the visual pathway caused by these diseases can potentially provide insights into treating other brain degenerative diseases. With relatively simple structure and tissue environment compared to the rest of the brain, it is possible that breakthroughs on CNS regeneration will be made easier in the visual system. We are trying to apply combinatorial strategies used during normal nervous system development, plus anti-inflammatory strategies to regenerate the visual pathway, in both rodent and non-human primate models of glaucoma and optic nerve injury. Similar strategies has been shown to successifully regenerate mouse spinal cord after injury, giving us more confidence in this research direction.
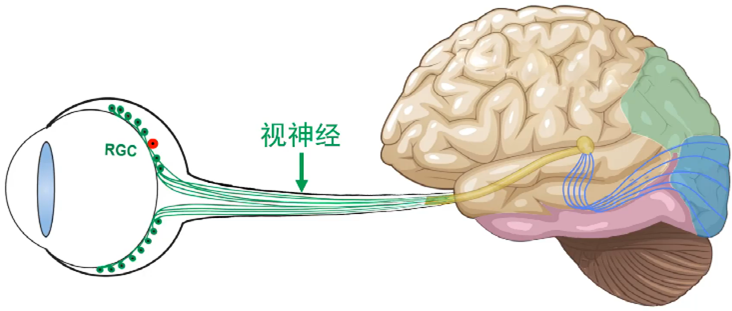
Image-forming visual pathway goes from retina to visual thalamus, then to visual cortex. Retinal ganglion cell degeneration and optic nerve injury cause lesion in the whole pathway and cannot be treated, posing a challenge to public health and economic development due to continuing increase of aged population in our country